SO3H-functionalized Brønsted acidic ionic liquids as efficient catalysts for the synthesis of isoamyl salicylate†
Abstract
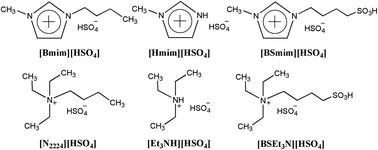
Six Brønsted acidic ionic liquids (BAILs) composed of [HSO4] were prepared, characterized, and used as catalysts of low dosage in the synthesis of isoamyl salicylate. The effects of various parameters such as the kind of BAILs, temperature, catalyst loading, and molar ratio of the reactants on the conversion of salicylic acid were also examined in detail. The results suggested that the catalytic performances of BAILs were of close relevance to their Hammett acidities. The SO3H-functionalized BAILs 1-(4-sulfonic acid) butyl-3-methylimidazolium hydrogen sulfate ([BSmim][HSO4]) and N-(4-sulfonic acid) butyl triethylammonium hydrogen sulfate ([BSEt3N][HSO4]) of strong acidities exhibited excellently catalytic activities and selectivities in the esterification of salicylic acid with isoamyl alcohol. The fully optimized geometries of [BSmim][HSO4] and [BSEt3N][HSO4] further manifest that their strong acidities are derived from the strong interactions between the anion with the sulfonic acid group. In addition, it was found that [BSmim][HSO4] could be also recovered easily and used repetitively at least six times without obvious decline in activity and quantity.

 Please wait while we load your content...
Please wait while we load your content...