Metal ion detection by naphthylthiourea derivatives through ‘turn-on’ excimer emission†
Abstract
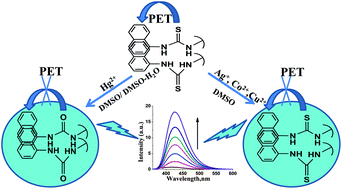
The detection of multiple heavy and transition metal (HTM) ions has been achieved through ‘turn-on’ excimer emission by three naphthylthiourea derivatives (L1, L2 and L3) in DMSO. Mechanistic studies suggest that the photoinduced electron transfer from the thiourea moiety to the naphthalene excimer is suppressed in the presence of Hg2+, Cu2+, Co2+ and Ag+, leading to enhanced emission intensity from the excimer. Interestingly, the ligands were able to detect all the above said HTM ions through ‘turn-on’ fluorescence, at micromolar concentrations of the analytes. While Hg2+ and Cu2+ ions were detected by all the ligands, Co2+ and Ag+ ions were detected only by L2 and L3, respectively. Furthermore, the detection of Hg2+ ions by L1 has been demonstrated in aqueous condition, wherein the fluorescence quantum yield showed the highest value (0.25 ± 0.01), compared to other metal–ligand complexes in DMSO. To the best of our knowledge, this is the first report regarding multi-ion detection by thiourea based ligands through ‘turn-on’ excimer emission.

 Please wait while we load your content...
Please wait while we load your content...