Bisindolines from the reaction of 3,5-dimethoxyaniline with vicinal diones†
Abstract
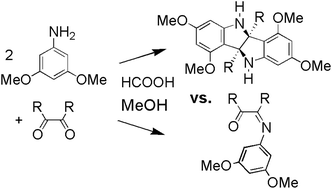
Substituted bisindolines were synthesized from the reaction of 3,5-dimethoxyaniline and vicinal diones (2,3-butanedione, benzil, and 4-methylbenzil). The reaction of 3,5-dimethoxyaniline with 2,3-butanedione under polar acidic conditions proceeded with an unanticipated double cyclization reaction forming 2,2′-dimethyl-5,5′,7,7′-tetramethoxybisindole, (+/−)-1. The reaction of 3,5-dimethoxyaniline with benzil under similar conditions did not form a bisindoline, but instead formed cis-1,2-diphenyl-2-[(3,5-dimethoxyphenyl)imino]ethanone, 2. When the solvent, acid, and reaction temperature were changed for the reaction of 3,5-dimethoxyaniline with benzil, 3,3-diphenyl-4,6-dimethoxyindolin-2-one, 3 (an isomer of 2), and 2,2′-diphenyl-5,5′,7,7′-tetramethoxybisindole, (+/−)-4, were isolated as major and minor products, respectively. Similarly, the reaction of 3,5-dimethoxyaniline with 4-methylbenzil produced a 1 : 1 mixture of cis-1-(4-methylphenyl)-2-phenyl-2-[(3,5-dimethoxyphenyl)-imino]ethanone, 6a, and cis-1-(phenyl)-2-(4-methylphenyl)-2-[(3,5-dimethoxyphenyl)imino]ethanone, 6b, under polar acidic conditions and 3-(4-methylphenyl)-3-phenyl-4,6-dimethoxyindolin-2-one, (+/−)-7 and 2-(4-methylphenyl)-2′-phenyl-5,5′,7,7′-tetramethoxybisindole, (+/−)-8, under apolar acidic conditions at elevated temperature. We propose the mechanism of bisindoline and indolinone formation to include a Friedel–Crafts type electrophilic attack on the electron rich aryl ring at the ring closing C–C bond forming steps, and further propose a common carbocationic monocyclized intermediate.

 Please wait while we load your content...
Please wait while we load your content...