A highly efficient and green cascade synthesis of 3-methyl-substituted-4-hydroxy-1-methyl-quinolin-2(1H)-ones under solvent- and catalyst-free conditions†
Abstract
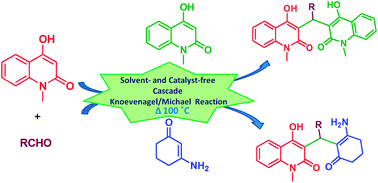
An expeditious and green protocol for the synthesis of 3,3′-methanediylbis(4-hydroxy-1-methylquinolin-2(1H)-one) (MDBHQ) derivatives and 3-((2-amino-6-oxocyclohex-1-enyl)methyl)-4-hydroxy-1-methylquinolin-2(1H)-one (AOCHQ) derivatives has been developed via a cascade Knoevenagel–Michael reaction. The products were obtained in high yield under neat reaction conditions without any work-up or chromatographic purification. The generality and functional tolerance of this environmentally benign protocol is demonstrated.

 Please wait while we load your content...
Please wait while we load your content...