Nickel ferrite as a stable, high capacity and high rate anode for Li-ion battery applications†
Abstract
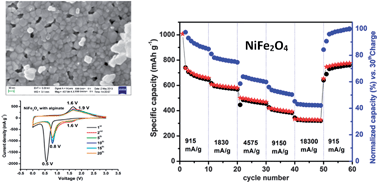
Conversion electrodes based on binary or ternary metal oxides are known to suffer from a slow electrode kinetic problem that is related to solid-state lithium diffusion, electrochemical stability and loss of coulombic efficiency in the initial cycles. The solid-state diffusion problem is mainly governed by the grain boundary effect during the conversion reaction but the reason behind the electrochemical stability is still unknown. To overcome the issues related to solid-state diffusion, electrodes are nanoarchitectured and made in nano-dimensions but it is still not clear how to solve the stability problem, which is very acute when electrodes perform at a high rate. To attempt solving this problem for the ternary based conversion anode, we have carried out this study. The current study describes the use of a nanosized NiFe2O4-based conversion anode along with an alginate binder as an excellent high rate (for instance, 20 C rate) electrode. In this study, more than 98% of the initial charge storage capacity of a nickel ferrite electrode is restored over a large number of cycles. Galvanostatic tests on such electrodes show that NiFe2O4 nanoparticles can deliver 740 mA h g−1 capacity at 1 C current rate with very good capacity retention. The observed outstanding electrochemical performance, stability and coulombic efficiency will help us to design a ternary metal oxide based electrode as an anode for future generation lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...