Efficient blue-emitting electrophosphorescent organic light-emitting diodes using 2-(3,5-di(carbazol-9-yl)phenyl)-5-phenyl-1,3,4-oxadiazole as an ambipolar host†
Abstract
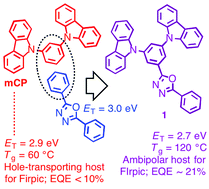
2-(3,5-Di(carbazol-9-yl)phenyl)-5-phenyl-1,3,4-oxadiazole, 1, was synthesised from the reaction of 3,5-di(carbazol-9-yl)benzohydrazide and trimethyl orthobenzoate at 185 °C. Compound 1 exhibits a glass-transition temperature of 120 °C, a reversible reduction at a half-wave potential of −2.34 V vs. ferrocenium/ferrocene, and an adiabatic triplet energy of 2.73 eV. Organic light-emitting diodes were fabricated using: solution-processed poly[6-(9H-carbazol-9-yl)-9-(4-vinylbenzyl)-9H-3,9′-bicarbazole] as the hole-transport layer; a vacuum-deposited emissive layer composed of 1 as a host and bis[(4,6-di-fluorophenyl)pyridinato-N,C2′](picolinato-N,O)iridium or fac-tris(2-phenylpyridinato-N,C2′)iridium as blue- or green-emitting phosphorescent guest molecules, respectively; and vacuum-deposited 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline or 3-([1,1′-biphenyl]-4-yl)-5-(4-(tert-butyl)phenyl)-4-phenyl-4H-1,2,4-triazole as the electron-transport layer. External quantum efficiencies of up to 21 and 25% were obtained for blue- and green-emitting devices, respectively.

 Please wait while we load your content...
Please wait while we load your content...