Conformation aspect in the α-amylase induced agglomeration of citrate-stabilized gold nanoparticles†
Abstract
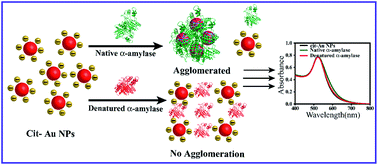
In this article we report the effect of a chemical denaturant on the agglomeration behaviour of a citrate stabilized Au NP–protein composite. We have used α-amylase as a model protein and urea as the chemical denaturant. The agglomeration behaviour has been studied by UV-Visible and Fourier transform infrared (FTIR) spectroscopy, circular dichroism (CD), dynamic light scattering (DLS) based particle size analyses, fluorescence studies, zeta potential measurements and transmission electron microscopy (TEM). Our studies indicate that when α-amylase was added to a cit–Au NP dispersion, agglomerated structures were formed whose sizes increased with time. On the other hand, when urea was also added, the agglomerated structures did not grow further indicating that the agglomeration process was arrested. In addition, urea was found to permeate to the surface of Au NPs in the agglomerated units, as seen by changes in the UV-Vis spectra, zeta potential measurements, FTIR and fluorescence measurements. Results of CD and FTIR studies are indicative of the α-helix of the protein being stabilized in the cit–Au NP induced conformational changes of α-amylase leading to its agglomeration. The activity of the proteins present in the agglomerated structures was still retained. Interestingly, addition of chemically denatured protein (urea treated α-amylase) to the cit–Au NP–protein composite did not result in further agglomeration of the composite. The observations reported herein indicate that the interaction between the cit–Au NP and the protein leading to agglomeration is dependent on the conformation of the protein, and that while native protein was involved in the agglomeration process, denatured protein was not involved in agglomeration process. Urea induced unfolding of the free proteins as well as the presence of ions in the medium stopped or stalled the growth of the agglomerated structures.

 Please wait while we load your content...
Please wait while we load your content...