Modular synthesis, spectroscopic characterization and in situ functionalization using “click” chemistry of azide terminated amide containing self-assembled monolayers†
Abstract
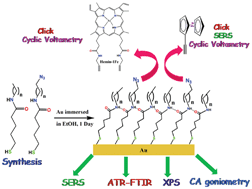
A general and modular synthetic route is developed for the synthesis of azidoalkylthiol molecules, which contain an

 Please wait while we load your content...
Please wait while we load your content...