Extraction of thorium from LiCl–KCl molten salts by forming Al–Th alloys: a new pyrochemical method for the reprocessing of thorium-based spent fuels
Abstract
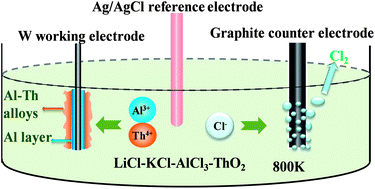
The co-reduction process of Th(IV) and Al(III) ions in the LiCl–KCl–AlCl3–ThO2 molten salts with different concentrations of AlCl3 was investigated at 800 K on the tungsten electrode. Cyclic voltammetry, square wave voltammetry and open-circuit chronopotentiometry techniques were used to study electrochemical behaviors of Al(III), Th(IV) and Al–Th alloy formation processes. The results showed that five kinds of Al–Th intermetallic compounds could be formed in the AlCl3 poor system, whereas only one kind of Al–Th compound formed in the AlCl3 rich system. Al–Th alloys were obtained by potentiostatic electrolysis on aluminum electrodes and by galvanostatic electrolysis on a tungsten electrode. All the Al–Th alloys were characterized by scanning electron microscopy (SEM) with energy dispersive spectrometry (EDS), X-ray diffraction (XRD) and inductively coupled plasma atomic emission spectrometry (ICP-AES). The XRD results showed that the Al–Th compound prepared in the AlCl3 rich system was Al3Th.

 Please wait while we load your content...
Please wait while we load your content...