Discovery of a pair of diastereomers as potent HDACs inhibitors: determination of absolute configuration, biological activity comparison and computational study†
Abstract
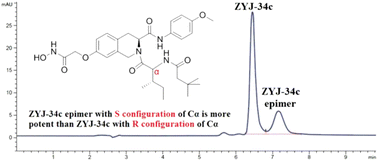
Histone deacetylase inhibitors (HDACi) are still the focus of epigenetic modulator development due to their effective intervention in many pathological processes. In our previous research, a potent HDACi was designed, synthesized and validated as a promising antitumor candidate named ZYJ-34c. Enlarged scale synthesis of ZYJ-34c for further detailed research was hindered by the occurrence of a by-product, which was identified as an isomer of ZYJ-34c by HRMS and 1H NMR. Subsequent synthesis route modification and optimization revealed that these two isomers were a pair of epimers and their absolute configurations could be directly determined by our optimized synthesis routes, through which each optically pure epimer could be stereoselectively synthesized, respectively. Based on these results, we concluded that our previously reported absolute configuration of ZYJ-34c was incorrect. It is worth noting that the epimer of ZYJ-34c exhibited more potent HDACs inhibition and both in vitro and in vivo antitumor activities, and moreover, their different HDACs inhibitory activities could be rationalized by computational simulations of their binding modes in HDAC2.

 Please wait while we load your content...
Please wait while we load your content...