Thermotropic and glass transition behaviors of n-alkyl β-d-glucosides†
Abstract
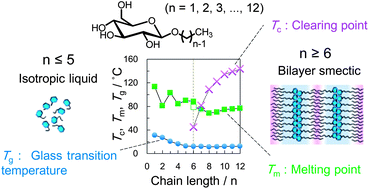
Alkyl β-D-glucosides (CnGlu) with different alkyl chain lengths (n = 1, 2, 3,…, 12) were prepared and the influence of the hydrophobic chain length on their thermotropic and glass transition behaviors was studied using differential scanning calorimetry (DSC), polarizing optical microscopy (POM) and X-ray diffraction analysis (XRD). In the heating process of crystalline CnGlu (n ≥ 7), two distinct transitions between the solid and the lamellar liquid crystalline (LC) phase at melting point (Tm) and between the LC phase and the isotropic liquid (IL) at the clearing point (Tc) were observed. Tc of C6Glu was observed monotropically at lower temperature than Tm. When the non-crystalline alkyl glucosides were rapidly cooled, the glass transition was ascertained for all glucosides. In the case of CnGlu (n ≥ 6) a glassy LC phase, the glass phase having anisotropy like lamellar phase, was formed. The glass transition temperature (Tg) lowered until the chain length reached C5 because of the reduction of the hydrogen bonding networks, while further increase of the chain length did not affect Tg irrespective of the of number of the hydrophobic chain length (n ≥ 5). When CnGlu was supercooled at room temperature, which is between Tm and Tg, XRD measurements showed that there was no structural difference in the glucose moiety working as a hydrophilic group in the resultant LC phase. It indicates the importance of the hydrogen bonding between the hydrophilic sugar moieties on the glass transition behavior of n-alkyl β-D-glucosides.

 Please wait while we load your content...
Please wait while we load your content...