The roles of betaine-ester analogues of 1-N-alkyl-3-N′-methyl imidazolium salts: as amphotropic ionic liquid crystals and organogelators†
Abstract
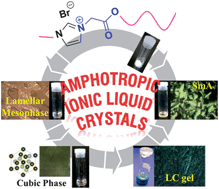
Betaine-ester analogues of 1-N-alkyl-3-N′-methyl imidazolium salts exhibiting both thermotropic and lyotropic liquid crystal behaviors are reported. Two typical compounds, structurally determined by single crystal X-ray diffraction exhibit an interdigitated bilayer packing. These compounds are gelators, which form gels in a variety of organic solvents such as chloroform, methanol, ethanol, tetrahydrofuran, while also exhibiting a lamellar mesophase. Results from IR and variable-temperature 1H NMR spectroscopy studies show that a trace amount of water (0.3–0.5% by volume) in CHCl3 plays a crucial role for developing the H-bonded network during gel formation. Ag-NPs (∼5–10 nm size) obtained from lyotropic LC solution and LC gel exhibit different morphologies ranging from spherical to truncated triangular plates.

 Please wait while we load your content...
Please wait while we load your content...