Total synthesis of 5-epi-Torrubiellutin C and its biological evaluation†
Abstract
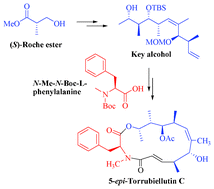
The total synthesis of 5-epi-Torrubiellutin C is described in a fully stereocontrolled manner and linear sequence involving high yielding steps. The synthetic strategy involves the dicyclohexylboron chloride mediated Paterson's aldol protocol, Horner–Emmons olefination, TiCl4 mediated syn-aldol reaction and ring closing metathesis (RCM) as the key steps. Furthermore, the resultant epimer was evaluated for cytotoxicity against DU145 (prostate), MCF-7 (breast) and A549 (lung) cancer cell lines, and the results showed that the 5-epi-Torrubiellutin C is as good as the natural product in bioassays.

 Please wait while we load your content...
Please wait while we load your content...