Diastereoselective 1,3-dipolar cycloadditions of both electronically modified phenyl-nitrile oxides and stilbenes†
Abstract
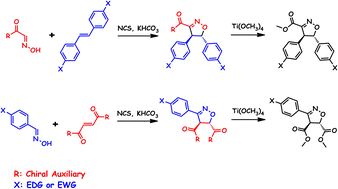
Menthyl carboxyloyl nitrile oxide addition is less selective for (E)-stilbene (12% d.e.) than for its corresponding (1R)-8-phenyl-menthyl (38% d.e.) or (2R)-bornane[10,2]sultam (48% d.e.) analogues. This lower selectivity is also observed when the chiral auxiliary is placed on the dipolarophile, as demonstrated by the [3 + 2] cycloadditions of p-NO2-phenyl nitrile oxide to acryloyl derivatives of (1R)-menthol (4% d.e.) and (2R)-bornane[10,2]sultam (60% d.e.). We managed to increase these diastereoselectivities by taking advantage of the Tolbert and Ali co-operative influence of two prosthetic groups, as seen in phenyl nitrile oxide addition to the bis-fumaroyl derivatives of (1R)-menthol (30% d.e.) and (2R)-bornane[10,2]sultam (98% d.e.). In the specific case of the N-acryloyl bornane[10,2]sultam, we found evidence for a small predictable negative influence of electronically deficient para-substituted phenyl nitrile oxides on diastereoselectivity (p-Me2N, 72% d.e.; p-F, 65% d.e.; p-NO2, 60% d.e.). This may be explained by the participation of the more reactive but thermodynamically less stable syn-s-cis conformer in the reaction pathway, given its smaller difference of calculated energies in the corresponding transition states. Such an explanation is further supported by the negative influence of either a polar solvent, stabilizing these more polar transition states (phenyl nitrile oxide in hexane, 76% d.e.; CH2Cl2, 71% d.e.; MeCN, 67% d.e.), or a chelating Lewis acid (MgCl2, 66% d.e.).

 Please wait while we load your content...
Please wait while we load your content...