A one-pot three-component domino protocol for the synthesis of penta-substituted 4H-pyrans†
Abstract
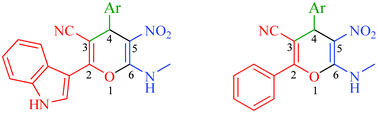
A library of novel 2-(1H-indol-3-yl)-6-(methylamino)-5-nitro-4-aryl-4H-pyran-3-carbonitriles and 6-(methylamino)-4-(aryl)-5-nitro-2-phenyl-4H-pyran-3-carbonitriles were synthesized in excellent yields via domino one-pot three-component reactions of

 Please wait while we load your content...
Please wait while we load your content...