Synthesis of monodispersed α-FeOOH nanorods with a high content of surface hydroxyl groups and enhanced ion-exchange properties towards As(v)†
Abstract
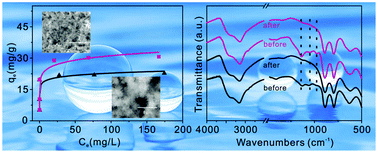
Monodispersed α-FeOOH nanorods, with a high content of surface hydroxyl groups, were synthesized using a simple solution method at room temperature. The obtained α-FeOOH nanorods were characterized by field emission scanning electron microscopy, transmission electron microscopy, thermal gravimetric analysis, nitrogen adsorption–desorption isotherms and X-ray diffraction. The presence of cetyltrimethylammonium bromide (CTAB) successfully avoids the congregation of the α-FeOOH nanorods, which resulted in the formation of the monodispersed α-FeOOH nanorods and the increase of the surface hydroxyl groups. The adsorption properties of the nanorods towards toxic As(V) ions were investigated. The results suggest that the adsorption properties of the monodispersed α-FeOOH nanorods were also improved compared with the congregated ones synthesized without CTAB or surfactant instead of pure water. The enhanced adsorption properties were attributed to the high content of the surface hydroxyl groups, which were revealed by X-ray photoelectron spectrometer and Fourier transform infrared absorption spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...