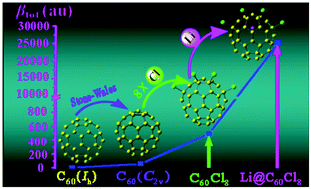
Recently, C60Cl8 (C2v) has been experimentally synthesized (Y.-Z. Tan, et al., Nat. Mater., 2008, 7, 790) by the addition of eight chlorine atoms to C60 (C2v), which is associated with a Stone–Wales transformation of C60 (Ih). In this work, the first hyperpolarizabilities (βtot) of C60 (C2v) and C60Cl8 (C2v) are investigated. After the Stone–Wales transformation and chlorine addition reaction, the βtot values slightly increase from 0 for C60 (Ih) to 60 au for C60 (C2v) and 502 au for C60Cl8 (C2v), respectively. To further enhance the first hyperpolarizability, the endohedral fullerene derivative, Li@C60Cl8, formed by encapsulating a lithium (Li) atom inside the C60Cl8, has been designed. Interestingly, the electron transfer between Li and C60Cl8 leads to an extremely large βtot value of 25 569 au, which is considerably larger (51 times) than the 502 au of C60Cl8. It shows that the encapsulated Li effect plays an important role in enhancing the first hyperpolarizability, so the Li@C60Cl8 can be considered as a candidate for high-performance nonlinear optical materials.

 Please wait while we load your content...
Please wait while we load your content...