Lactam/MoCl5 interaction in CH2Cl2: synthesis and X-ray characterization of protonated δ-valerolactam salts†
Abstract
The protonated 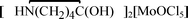 , 1,
, 1, 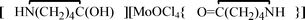 , 2, and
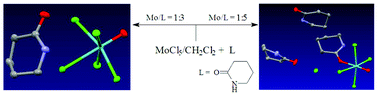
, 2, and 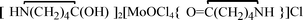 , 3, were isolated from the non selective reaction of MoCl5 with δ-valerolactam in
, 3, were isolated from the non selective reaction of MoCl5 with δ-valerolactam in 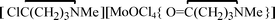 , 4, in 74% yield, together with minor amount of
, 4, in 74% yield, together with minor amount of 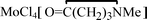 , 5. The products 1–5 were characterized by analytical and spectroscopic techniques, and by
, 5. The products 1–5 were characterized by analytical and spectroscopic techniques, and by

 Please wait while we load your content...
Please wait while we load your content...