Functionalization of BODIPY dyes at 2,6-positions through formyl groups†
Abstract
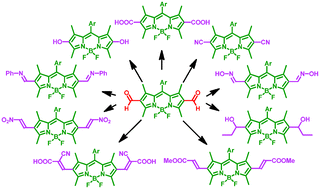
A 2,6-diformyl-BODIPY dye has been modified by transforming its formyl groups at the 2,6-positions into different functional groups such as hydroxyl, carboxylic acid, cyano, nitro and oxime groups, resulting in a series of new BODIPY dyes. The optical properties of the resulting BODIPY dyes significantly depend on the functional groups at the 2,6-positions.

 Please wait while we load your content...
Please wait while we load your content...