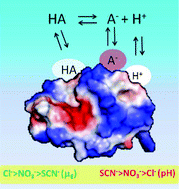
Ion specificity is a long standing unsolved puzzle of chemistry and biology. Myriad experiments underline its universality. We have studied ion specific effects on different buffers at the same nominal bulk pH. We used both electrophoretic light scattering and pH measurements to investigate a system constituted by lysozyme dissolved in different buffer solutions (TrisHCl, carbonate, cacodylate, phosphate, citrate). Each buffer had the same initial nominal pH (7.15). The experiments were done in the presence of three sodium salts (NaCl, NaNO3, NaSCN). The results are unequivocal. We found that buffer type strongly affects electrophoretic mobility and the pH of salt–lysozyme solutions. The role played by the buffer is as important as that played by the different sodium salts. We also investigated striking additional co-ion specific effects due to the substitution of sodium with potassium. The observed effects due to an interplay among ion specificity, pH and buffer type have major implications not previously recognised.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...