Isomers of C12N12 as potential hydrogen storage materials and the effect of the electric field therein†
Abstract
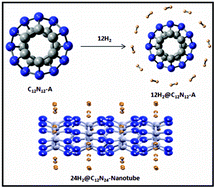
The relative stability, aromaticity and hydrogen storage ability of different C12N12 isomers are computationally assessed at B3LYP/6-31G(d), DFT-D-B3LYP/6-31G(d) and M052X/6-311+G(d,p) levels of theory. A negative

 Please wait while we load your content...
Please wait while we load your content...