Interplay between hydroxyl radical attack and H-bond stability in guanine–cytosine†
Abstract
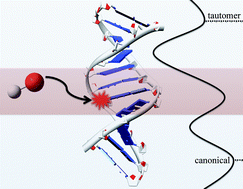
We combine ONIOM simulations with an elaborated hydrated DNA model to evaluate the effects of the hydroxyl radical, one of the major products in irradiated cells, on the stability of the guanine–cytosine (G:C) base pair. Our results demonstrate that the addition of the hydroxyl radical to the cytosine moiety results in inter-base hydrogen bonds rearrangements, eventually leading to a so-called rare tautomeric isomer. In these processes, the O6(G)–N4(C) hydrogen bond strength plays a crucial role as one of the driving forces for the double proton transfer. We also demonstrate that the surrounding environment affects the stability of the non-canonical mutagenic forms. As in undamaged DNA, the

 Please wait while we load your content...
Please wait while we load your content...