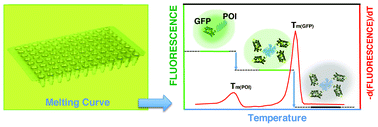
The development of differential scanning fluorimetry and the high-throughput capability of Thermofluor have vastly facilitated the screening of crystallization conditions of proteins and large mutant libraries in structural genomics programs, as well as ligands in drug discovery and functional genomics programs. These techniques are limited by their requirement for both highly purified proteins and solvatochromic dyes, fueling the need for more robust technologies that can be used with crude protein samples. Here, we present the development of a new high-throughput technology for the quantitative determination of protein stability and ligand binding by differential scanning fluorimetry of GFP-tagged proteins. This technology is based on the principle that a change in the proximal environment of GFP, such as unfolding and aggregation of the protein of interest, is measurable through its effect on the fluorescence of the fluorophore. Protein stability data was generated for twelve GFP-tagged proteins including monomeric and multimeric, DNA-binding, RNA-binding, proteolytic, heat-shock and metabolic proteins of Escherichia coli, Burkholderia pseudomallei, Staphylococcus aureus, dengue and influenza (H5N1) viruses. The technology is simple, fast and insensitive to variations in sample volumes, and the useful temperature and pH range is 30–80 °C and 5–11 respectively. The system does not require solvatochromic dyes, reducing the risk of interference. The protein samples are simply mixed with the test conditions in a 96-well plate and subjected to a melt-curve protocol using a real-time thermal cycler. The data are obtained within 1–2 h and include unique quality control measures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...