Evaluation on the role of terminal N-substitution in 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde thiosemicarbazones on the biological properties of new water-soluble nickel(ii) complexes†
Abstract
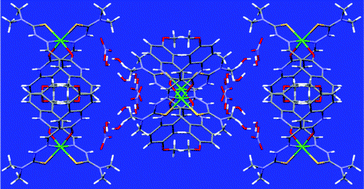
Five new nickel(II) complexes of 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde 4N-substituted thiosemicarbazones with the general formula {[Ni(HL)2](NO3)2·4H2O} have been synthesised in order to ascertain the biological properties due to the change in substitution at terminal nitrogen of the thiosemicarbazones. The structure of one of the complexes was established by single-crystal X-ray diffraction analysis. DNA/protein interactions of the complexes have been examined by photophysical studies which revealed that all the complexes can bind with calf thymus DNA via intercalation and the complexes bind to bovine serum albumin more strongly. Antioxidant studies showed that the Ni(II) complexes have significant antioxidant activity against 2-2′-diphenyl-1-picrylhydrazyl radical and 2,2′-azino-3-ethylbenzthiazoline-6-sulfonic acid diammonium salt cation radical. The in vitro cytotoxicity of all the complexes against the A549 cell line was assayed; this showed highest cytotoxic activity for the complex containing phenyl substituted thiosemicarbazone – higher than found for cisplatin.

 Please wait while we load your content...
Please wait while we load your content...