DEMS-monitoring liquid | gas interfacial ammonia oxidation at carbon nanofibre membranes
Abstract
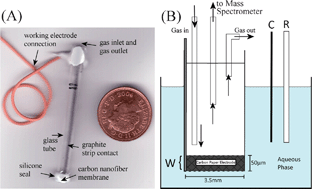
Electrochemical ammonia oxidation is of interest in waste treatment as well as in electrochemical sensing applications and demonstrated here at a carbon nanofibre (“bucky-paper”) electrode. The electrode is placed at the aqueous electrolyte | gas interface, and current (cyclic voltammetry) as well as ambient differential electrochemical mass spectrometry (DEMS, cyclic voltbarometry) data are recorded as a function of solution composition and pH. The oxidation of oxalate to CO2 is employed as a test and calibration system. Anodic polarization of the carbon nanofibre membrane in inert aqueous electrolyte is shown to result in direct sustained anodic CO2 evolution. In alkaline aqueous media (starting at pH 9) significant levels of nitrogen from ammonia are produced in competition to CO2 formation from carbon nanofibres without the need for additional catalysts. However, for applications with low level ammonia, catalysts will be required to minimize current losses, carbon nanofibre corrosion, and side product formation.

 Please wait while we load your content...
Please wait while we load your content...