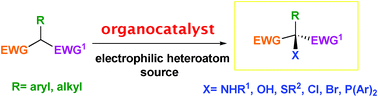
The enantioselective α-heterofunctionalization of α-substituted 1,3-dicarbonyl compounds is an attractive area of organic chemistry, thanks to the undoubted value of the final compounds as versatile intermediates for the synthesis of natural products and pharmaceutical targets. In this context, the organocatalytic approach is gaining importance, alongside the asymmetric metal-based catalysis, for the production of molecules with highly functionalized chiral quaternary stereocenters containing a carbon-heteroatom bond. This review aims to illustrate progress in the enantioselective α-amination, α-hydroxylation, α-sulfenylation and α-halogenation reactions of α-substituted 1,3-dicarbonyl and related compounds mediated by mono-, bi-, multi-functional organocatalysts, phosphoric acids and phase transfer catalysts appeared from 2002 to mid-2011.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...