Brønsted-acid catalyzed condensation-Michael reaction-Pictet–Spengler cyclization—highly stereoselective synthesis of indoloquinolizidines†
Abstract
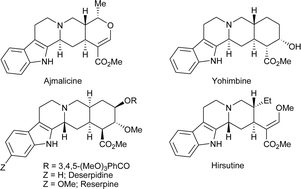
A highly diastereoselective methodology for the efficient synthesis of functionalized indolo[2,3-a]quinolizidine skeletons in a one-pot operation has been developed. The protocol makes use of simple and inexpensive starting materials such as

 Please wait while we load your content...
Please wait while we load your content...