Visible-light-mediated radical arylthiodifluoromethylation of isocyanides with fluorinated 2-pyridyl sulfones†
Abstract
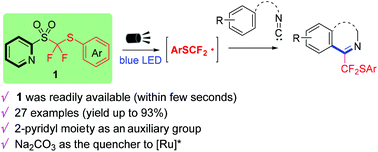
A novel class of shelf-stable and easily scalable arylthiodifluoromethyl 2-pyridyl sulfone derivatives was developed as powerful arylthiodifluoromethylation reagents, which allow efficient radical arylthiodifluoromethylation of various isocyanides to form phenanthridines and isoquinolines in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...