1,4-Refunctionalization of β-diketones to γ-keto nitriles via C–C single bond cleavage†
Abstract
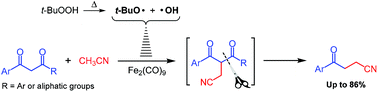
The 1,4-refunctionalization of β-diketones to γ-keto nitriles was realized via an Fe-catalyzed cascade radical process. The C–C single bond cleavage occurred at the less sterically hindered alkyl ketone side or at the side of β-diarylketones with electron-withdrawing groups. The reaction mechanism was proposed with respect to ESI-MS analysis results.


 Please wait while we load your content...
Please wait while we load your content...