One-pot, highly efficient, cavity controllable synthesis and binding properties of carbazole-based macrocycles with sulfonamide linkages†
Abstract
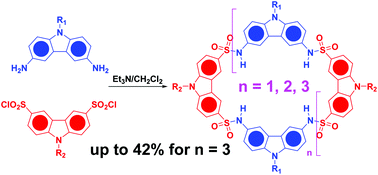
A new type of carbazole-based macrocycle with different dimensions (up to eight carbazole units) was synthesized via formation of sulfonamide linkages. A high cyclization efficiency was achieved under normal synthetic conditions. By introduction of different periphery tails on the carbazole precursors, the product distribution could be substantially adjusted. Moreover, products with specific dimensions could be selectively obtained through a fragment coupling strategy. All the macrocycles showed strong complexation towards the 2,5-dimethoxyterephthalate guest with a 1 : 1 stoichiometry. The binding constants were determined to be of the order of magnitude of 104via UV-vis and FL titrations.


 Please wait while we load your content...
Please wait while we load your content...