The serendipitous discovery of a readily available redox-bistable molecule derived from cyclic(alkyl)(amino)carbenes†
Abstract
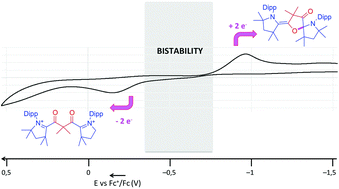
Bis(azoliums) are readily available in one step from cyclic (alkyl)(amino)carbenes and bis(acyl chlorides). A two-electron reduction of the bis(azolium), featuring a gem-(dimethyl)malonoyl spacer, leads to the corresponding transient diradical, which undergoes an intramolecular cyclization. The latter can be re-oxidized at a higher potential to yield back the bis(azolium). The redox bistability of this simple organic molecular system is linked to the formation of a weak C–O bond (27 kcal mol−1). Both redox forms can be isolated and stored for months without evidence of decay.


 Please wait while we load your content...
Please wait while we load your content...
