Visible light-induced aryltrifluoromethylation of hydroxy alkenes via radical trifluoromethylation-triggered aryl and heteroaryl migration†
Abstract
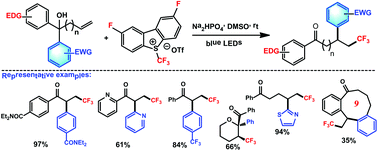
An efficient protocol was developed to achieve the aryltrifluoroalkylation of hydroxy alkenes via radical trifluoromethylation-triggered aryl migration. This method possesses several interesting features: (I) visible light is used as the sole promoter; (II) a new version of Umemoto's reagent [2,8-difluoro-S-(trifluoromethyl)dibenzothiophenium triflate], which is stable, cheap and recyclable, serves as the CF3 source; (III) the aryl migration is chemoselective; (IV) structurally diverse trifluoromethyl ketones can be produced.


 Please wait while we load your content...
Please wait while we load your content...