Total synthesis of (−)-akaol A via a conformational constraint strategy†
Abstract
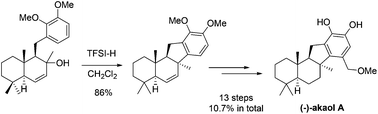
The enantioselective total synthesis of (−)-akaol A has been achieved. The key reaction features a well-designed Friedel–Crafts cyclization, which ensured a cis-fused B/C ring through a conformational constraint strategy. In marked control to trans-fused B/C ring cyclization promoted by a routine acid, the complete cis selective formation of the B/C ring was realized for the first time. Bistri-fluoromethane-sulfonimide promoted the key cyclization effectively. Our synthesis involves 13 steps from known aldehyde 2 and proceeds in 10.7% overall yield.


 Please wait while we load your content...
Please wait while we load your content...