Palladium-catalysed coupling of α-halo vinylphosphonate and α-phosphonovinyl sulfonate with alkylzincs: straightforward and versatile synthesis of α-alkyl vinylphosphonates†
Abstract
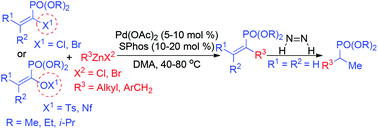
With α-(pseudo)halo vinylphosphonate as the electrophilic coupling partner, a variety of alkyl- and benzylic zinc reagents underwent vinylation reactions to produce α-alkyl vinylphosphonates. This unprecedented Pd-catalysed Negishi coupling features mild reaction conditions, high functional group tolerance, broad substrate scope, retention of the olefin geometry, and easy gram-scale synthesis.


 Please wait while we load your content...
Please wait while we load your content...