Organocatalytic asymmetric synthesis of cornolactones A and B, and formal synthesis of brasoside and littoralisone†
Abstract
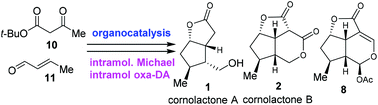
The asymmetric total synthesis of cornolactones A and B as well as the formal asymmetric synthesis of brasoside and littoralisone were accomplished in short steps, from simple starting materials. The key features of the synthesis include an efficient organocatalytic access to cyclopentenal 15, an intramolecular Michael addition reaction and an intramolecular oxa-Diels–Alder reaction.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...