Total synthesis of orientalol F via gold-catalyzed cycloisomerization of alkynediol†
Abstract
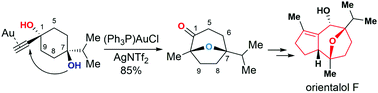
The total synthesis of orientalol F has been achieved starting from 1,4-dioxaspirodecan-8-one 11 in 13 steps. The key steps in this synthesis feature: (1) gold-catalyzed tandem cycloisomerization of alkynediol 10 for the formation of its seven-membered oxa-bridged bicyclic skeleton 9 of orientalol F, (2) visible-light-promoted organocatalytic aerobic oxidation of silyl enol ether 16 to enone 17, (3) Barbier-type butenylation for the diastereoselective synthesis of allylic alcohol 18 from enone 17, and (4) substrate-controlled Pd-catalyzed hydrogenation of 20 for the stereoselective installation of the C1 stereogenic center of 8.
- This article is part of the themed collection: Synthetic Approaches to Natural Products via Catalytic Processes


 Please wait while we load your content...
Please wait while we load your content...