Transition-metal-free radical tri-/difluoromethylation of N,N-dialkylhydrazones with sodium sulfinates†
Abstract
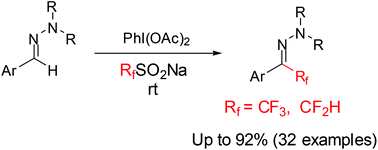
A novel protocol is herein described for the preparation of tri-/difluoromethylated N,N-dialkylhydrazones under transition-metal-free conditions and this reaction can be scaled up to the gram level easily. The reaction protocol is operationally simple and performed at ambient temperature, allowing obtaining the desired products in satisfactory yields. The easy-to-handle sodium tri- and difluoromethanesulfinates serve as effective fluoroalkyl radical precursors.


 Please wait while we load your content...
Please wait while we load your content...