Synthesis of 1,2,2-trifluorovinyl sulphides and selenides from trifluorovinylation of organic thiocyanates and selenocyanates†
Abstract
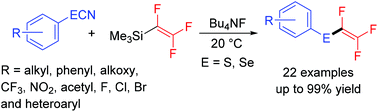
A nucleophilic trifluorovinylation of organothiocyanates catalyzed by tetrabutylammonium fluoride has been developed. In this transformation, a variety of aryl and alkyl thiocyanates reacted with trifluorovinyl trimethylsilane to afford 1,2,2-trifluorovinyl sulphides. This transformation was extended to organic selenocyanates to afford 1,2,2-trifluorovinyl selenides. The presented methodology is simple and mild, and can also be used to prepare 1,2,2-trifluorovinyl sulphoxides from organothiocyanates.


 Please wait while we load your content...
Please wait while we load your content...