Acid promoted Ir–P^N complex catalyzed hydrogenation of heavily hindered 3,4-diphenyl-1,2-dihydronaphthalenes: asymmetric synthesis of lasofoxifene tartrate†
Abstract
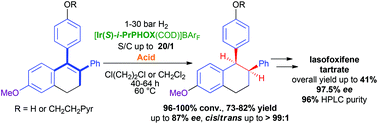
In this report we present the first successful homogenous asymmetric hydrogenation of heavily hindered and minimally functionalized tetrasubstituted cyclic olefins bearing three aromatic substituents, which represent key precursors of lasofoxifene tartrate. The success of our hydrogenation method is based on the surprising discovery that Brønsted acids or Lewis acids can significantly enhance the reactivity of these substrates towards hydrogenation with Ir–P^N complexes. Iterative screens of Ir–P^N catalysts, additives and reaction conditions led to high to full conversions with high ee. The obtained hydrogenation products are easily converted to optically pure selective estrogen receptor modulator lasofoxifene tartrate in significantly higher overall yield compared to previously reported methods.


 Please wait while we load your content...
Please wait while we load your content...