Pd-Catalyzed thiophene directed regioselective functionalization of arenes: a direct approach to multiply-substituted benzyl amines†
Abstract
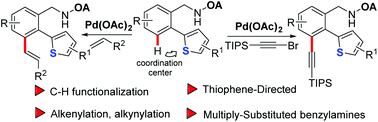
The first example of employing thiophene as the coordination center to assist C–H functionalization via a palladium catalyst has been described. The synthetically challenging penta-substituted benzylamines with five different functional groups could be easily achieved by taking advantage of transitive oxalyl amide/thiophene-directed C–H functionalization, which highlights its versatility in the construction of multiply-substituted arenes. The penta-substituted benzylamine displays its potential nature as a hole-transporting material.


 Please wait while we load your content...
Please wait while we load your content...