The study of metal-free and palladium-catalysed synthesis of benzochromenes via direct C–H arylation using unactivated aryl benzyl ethers derived from essential oils as raw materials†
Abstract
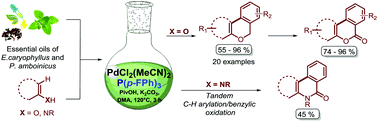
The synthesis of 6H-benzo[c]chromenes from unactivated 2-bromo aryl benzyl ethers was studied through two approaches: (i) the transition-metal-free intramolecular dehydrohalide coupling via intramolecular homolytic aromatic substitution; and (ii) the intramolecular cyclization via direct C–H arylation catalysed by PdCl2(MeCN)2. Having developed the most efficient method, a 17-membered chromene library was prepared in good yields and in shorter reaction times starting from commercially available phenol derivatives and phenol-rich essential oils (Eugenia caryophyllys and Plectranthus amboinicus) as raw materials, proving how sustainable and eco-friendly our protocol is. Additionally, the ruggedness of the optimized reaction conditions was evaluated with N-benzylanilines, giving the respective phenanthridone as an unexpected product, while the metal-free oxidation of the obtained 6H-benzo[c]chromenes was also performed to furnish the benzocoumarins.


 Please wait while we load your content...
Please wait while we load your content...