Oxidative trifluoromethylthiolation and thiocyanation of amines: a general approach to N–S bond formation†
Abstract
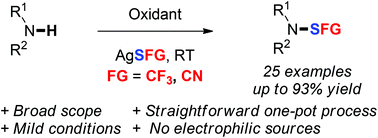
Herein, we described a practical and efficient one-pot oxidative trifluoromethylthiolation of amines. A panel of primary and secondary amines was functionalized in good yields at room temperature. This general approach was further applied to the thiocyanation of anilines and the corresponding products can be readily used as SCN electrophilic sources.

 Please wait while we load your content...
Please wait while we load your content...