C(sp3)–H bond functionalization by sequential hydride transfer/cyclization: electronic effect and steric effect controlled regioselectivity
Abstract
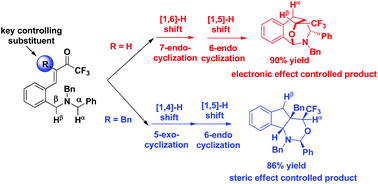
The electronic effect and steric effect have a dramatic impact on the fascinating cascade [1,n]-hydride transfer/cyclization. The regioselectivity of two potential hydrogen donors could be perfectly tuned to construct different skeletons.
- This article is part of the themed collection: 2016 Organic Chemistry Frontiers Review-type Articles

 Please wait while we load your content...
Please wait while we load your content...