Palladium-catalysed coupling reaction of aminals with N-sulfonyl hydrazones to give allylic sulfones†
Abstract
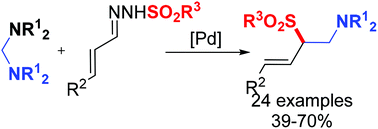
Palladium-catalysed cross-coupling of aminals with N-sulfonyl hydrazones has been established via C–N bond activation under base-free conditions, in which one C–C bond and one C–S bond were simultaneously generated. It was successfully applied in the construction of a variety of aminomethyl substituted allylic sulfones. Preliminary mechanistic studies indicated that the unique electrophilic cyclopalladated complex was involved in the catalytic cycle.
- This article is part of the themed collection: 2015 Emerging Investigators by OCF

 Please wait while we load your content...
Please wait while we load your content...