Direct transformation of amides: a one-pot reductive Ugi-type three-component reaction of secondary amides†
Abstract
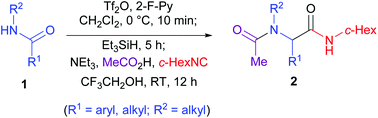
Amides are a class of highly stable carbonyl compounds, which are widely used as reliable synthetic intermediates in both organic synthesis and pharmaceutical chemistry. Thus, the direct transformation of amides into other classes of compounds under mild reaction conditions is highly desirable and in the meantime challenging. An efficient reductive Ugi-type reaction has been established. The method employs common secondary amides as starting materials. The reaction exhibited a broad substrate scope, good chemoselectivity and functional group tolerance and provides rapid access to a variety of functionalized α-acetamidoamides.

 Please wait while we load your content...
Please wait while we load your content...