Palladium catalyzed amide-oxazoline directed C–H acetoxylation of arenes†
Abstract
Herein we reported a Pd-catalyzed ortho-acetoxylation of arenes through a six-membered system using an amide-oxazoline as the directing group. A wide range of aryls and heteroaryls are tolerated. The approach provides general and straightforward access to phenol esters without the need for extra oxidants.
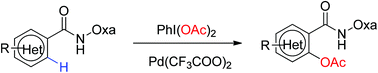

 Please wait while we load your content...
Please wait while we load your content...