Access to pyridines via DMAP-catalyzed activation of α-chloro acetic ester to react with unsaturated imines†
Abstract
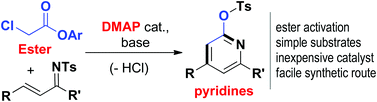
Facile access to trisubstituted pyridines from α-chloro acetic ester and unsaturated imines is achieved. DMAP-catalyzed activation of ester to form an enolate intermediate constitutes a key reaction step. On the application side, the wide availability and low cost of the substrates and catalysts make this method very attractive.

 Please wait while we load your content...
Please wait while we load your content...