An efficient halometallate ionic liquid functionalized mesoporous ZSM-5 for the reduction of carbon–carbon multiple bonds†
Abstract
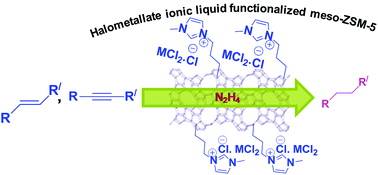
Transition metal-containing halometallate ionic liquids (ILs)-based economical catalysts have been developed for the selective reduction of carbon–carbon multiple bonds with hydrazine hydrate in ethanol under mild reaction condition. ILs have been tethered to the surface of mesoporous ZSM-5. The Mn-based halometallate IL tethered to the surface of mesoporous ZSM-5 exhibited the best activity compared to the parent halometallate ILs. It demonstrated efficient recyclability with no appreciable loss in the catalytic activity even after being recycled five times. In order to establish the reaction mechanism, ILs-hydrazine complexes were prepared and investigated in reduction reactions. The structure–activity relationship was established by their catalytic activities, physicochemical characterizations, ILs-hydrazine complex formation, and probe reactions. The catalyst also exhibited excellent activity in the reduction of alkynes to alkanes. This catalytic process demonstrated several key advantages such as mild and convenient reaction condition, low substrate to hydrazine ratio, reusability, and the cost-effectiveness of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...