FeMoO4 nanorod array: a highly active 3D anode for water oxidation under alkaline conditions†
Abstract
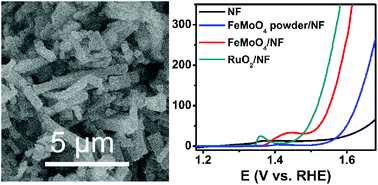
The development of efficient and durable oxygen evolution reaction (OER) catalysts for energy-saving electrolytic hydrogen generation is highly attractive. In this communication, we report the development of an FeMoO4 nanorod array on nickel foam (FeMoO4/NF) via a facile hydrothermal process. As a 3D oxygen evolution reaction electrocatalyst, such FeMoO4/NF shows superior catalytic activity with a geometrical catalytic current density of 50 mA cm−2 at an overpotential of only 293 mV in 1.0 M KOH. Notably, this electrode also shows good long-term electrochemical durability with a high turnover frequency value of 0.43 mol O2 s−1 at an overpotential of 390 mV. This study provides us an attractive cost-effective 3D catalyst material in water-splitting devices for efficient and durable water oxidation at alkaline pH.


 Please wait while we load your content...
Please wait while we load your content...