Luminescence and magnetocaloric effect of Ln4 clusters (Ln = Eu, Gd, Tb, Er) bridged by CO32− deriving from the spontaneous fixation of carbon dioxide in the atmosphere†
Abstract
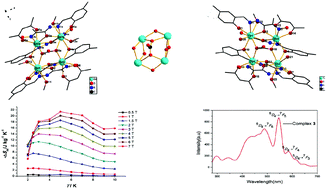
The synthesis and characterization of a new family of neutral tetranuclear Ln(III) complexes, [Eu4L4(CO3)(acac)2(H2O)2(CH3OH)2]·5H2O·2CH3OH (1), [Gd4L4(CO3)(acac)2 (CH3OH)4]·2CH3OH·CH2Cl2 (2), [Tb4L4(CO3)(acac)2(H2O)2(CH3OH)2]·3H2O·CH3OH (3), and [Er4L4(CO3)(acac)2(H2O)2(CH3OH)(DMF)]·H2O·CH3OH (4) (where acac− is acetylacetonate, H2L is 2-(hydroxyimino)-2-[(5-methyl-2-hydroxyphenyl)methylene]hydrazide and DMF = N,N-dimethyl formamide) are reported. For complexes 1–4, by absorbing and fixing CO2 spontaneously under atmospheric conditions, with CO32− as a bridging tetradentate ligand, the four LnIII ions are bridged by two μ2-O oxygen atoms from CO32− to form a Ln4 cluster. X-ray diffraction data reveal that the non-centrosymmetric (1, 3 and 4) or centrosymmetric (2) unit has two distinct LnIII ions, nine-coordinated LnIII ions in distorted spherical capped square antiprism geometry and eight-coordinated LnIII ions in distorted triangular dodecahedron geometry. Magnetic study indicates that complex 2 can act as a cryogenic magnetic refrigerant and the magnetocaloric effect (MCE) was detected with the magnetic entropy change of −ΔSm = 21.43 J kg−1 K−1 at ΔH = 7 T and 5 K. Furthermore, the fluorescence properties of complexes 1 and 3 were also investigated. The results prove that complex 3 can show TbIII characteristic emission peaks, while for complex 1, EuIII typical emission peaks were not observed.


 Please wait while we load your content...
Please wait while we load your content...